
Canopy Health Innovations, the research subsidiary of Canopy Growth (TSX:WEED; NYSE:CGC) that focuses on the development of therapeutic and natural health cannabis products, will get a big boost from a blockbuster $5-billion (Canadian) investment by Constellation Brands (NYSE:STZ) in Canopy Growth, a world-leading diversified cannabis and hemp company.
“Constellation Brands clearly has a strong interest in our research that supports the development of beverages containing cannabinoids, but that’s only the tip of the iceberg,” Marc Wayne, co-managing director of Canopy Health, says in an interview with BioTuesdays. “The additional capital is very beneficial. It will serve to expand and accelerate our other industry-leading research programs.”
Mr. Wayne says that in addition to informing innovative beverage development, Canopy Health is developing and conducting rigorous clinical programs to generate data to support broad global market access of its Spectrum branded medical line. Product-specific data also serves to enhance global IP protection for its cannabis-based products.

Mark Ware, chief medical officer for Canopy Growth and co-managing director of Canopy Health, points out that pain management, insomnia and mood-related disorders represent priority therapeutic areas for the company, explaining that “many of these overlap, so patients with chronic diseases may have issues with two or all three of these areas.”
Within pain disorders, Dr. Ware says that Canopy Health is conducting clinical trials in fibromyalgia and cancer pain, as well as general muscular and skeletal pain syndromes.
For consumer products, Canopy Health also applies the core principles of evaluating pharmacodynamics, dose response and safety to a range of potential products from beverages to topical and inhalation agents. “The principles of pharmacology are applied to products in all areas of development,” he adds.
Canopy Health has developed a unique ‘hub and spokes’ research model to facilitate and oversee global research activity. Canada acts as the central research ‘hub’ that monitors global research strategy, supply relationships, regulatory filings, product safety, scientific information and intelligence. As the ‘spokes’ of the model, Canopy Health has core research teams and regional operations in South America, Europe, UK, Africa and Australia that manage academic partnerships, regional IP, research sites and patient recruitment.
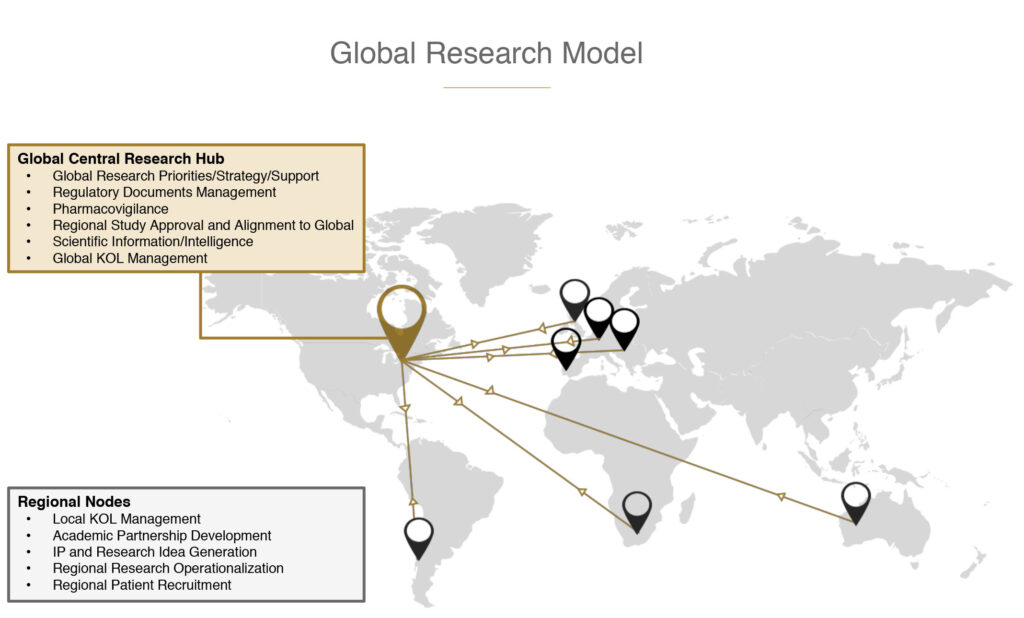
In addition, Canopy is one of the few companies developing cannabis-based drug and OTC products for companion animals through its affiliate, Canopy Animal Health, which is conducting Health Canada-approved trials with a focus on palliative care, anxiety, appetite stimulation and pain management. “We are working with top research organizations that have veterinary drug development experience. These animal studies also serve to support our work in humans. It’s highly strategic,” Mr. Wayne points out.
As part of its human therapeutic program, Canopy Health is investigating multiple formulations across what the company calls the “cannabis spectrum,” and how the ratio of THC-to-CBD, the two main active ingredients in cannabis, can be harnessed to develop products that improve patients’ lives.
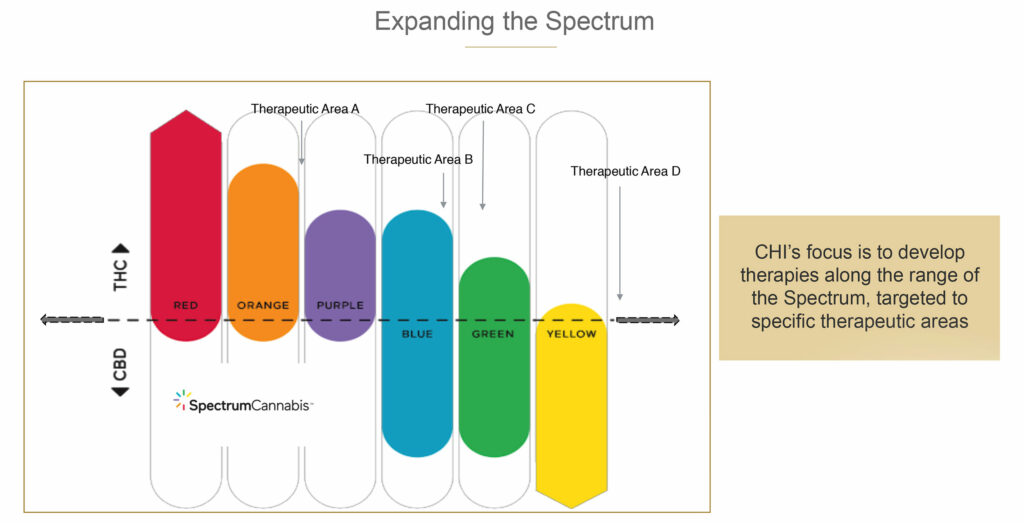
In its three lead placebo-controlled clinical trial programs, Canopy Health is assessing the safety and efficacy of a range of cannabinoid compounds in sleep, fibromyalgia and social anxiety disorder.
“These multicenter trials in Canada are some of the largest that have been conducted in these areas,” Dr. Ware contends. “We hope to take all three programs to the end of Phase 2b in the 2019-2020 time frame in order to establish dosing, safety and proof-of-concept efficacy. If the data are strong, we would not rule out the possibility of an external partnership for a Phase 3 trial, or we could proceed on our own,” he adds.
According to industry estimates, the global pain market is forecast to reach $77-billion by 2023, on a projected 4% compound annual growth rate between 2017 and 2023. In addition, the global insomnia and generalized anxiety disorder markets are expected to reach $5.5-billion and $7.5-billion, respectively, by 2023.
To support clinical drug development, Canopy Health is also conducting multiple Phase 1 studies for its therapeutic and consumer products to evaluate pharmacokinetic and pharmacodynamic data in healthy volunteers.
Outside of Canada, the company has partnered with British drug policy think-tank, The Beckley Foundation, to establish Beckley Canopy Therapeutics as a stepping-stone for Canopy Health’s expanded, European-focused research capacity.
Mr. Wayne says the British partnership aims to combine Canopy Growth’s cannabis expertise and supply with The Beckley Foundation’s strong research heritage and world class scientific partnerships to develop clinically validated and licensed cannabis-based medicines and natural health products, with a strong focus on intellectual property protection.
“The regulatory climate for starting cannabinoid clinical trials is extremely positive,” Dr. Ware points out. “Medical regulators are very interested in hearing from companies that are proposing serious and credible research with cannabis because this is what patients and the medical community is expecting.”
Citing the first FDA-approval of a cannabis-based drug, GW Pharmaceuticals’ Epidiolex for the treatment of childhood epilepsy, Dr. Ware says that as far as regulatory agencies are concerned, “it’s the science that does the talking.”
• • • • •
To connect with Canopy Health, or any of the other companies featured on BioTuesdays, send us an email at [email protected].






