
ImpediMed’s (ASX:IPD) transition to a software-as-a-service (SaaS) subscription business model in 2018, in conjunction with the introduction of the SOZO Digital Health Platform, is already paying off on the top line.
“We moved from a consumable and capital equipment business model to a high-margin subscription model where doctors pay us a flat fee on a monthly basis as they test their patients,” Richard Carreon, managing director and CEO, says in an interview with BioTuesdays.
Subscription revenue contributed 30% of the company’s medical revenue for the fiscal second quarter ended Dec. 31, 2018, and our future subscription revenue pipeline was $7-million at the end of the quarter, up 49% from the previous quarter. “This means we are seeing a strong customer and SaaS revenue pipeline building,” he adds.
ImpediMed is the world leader in the design and manufacture of medical devices employing next generation bioimpedance spectroscopy (BIS) technologies for use in the non-invasive clinical assessment and monitoring of tissue composition and fluid status.
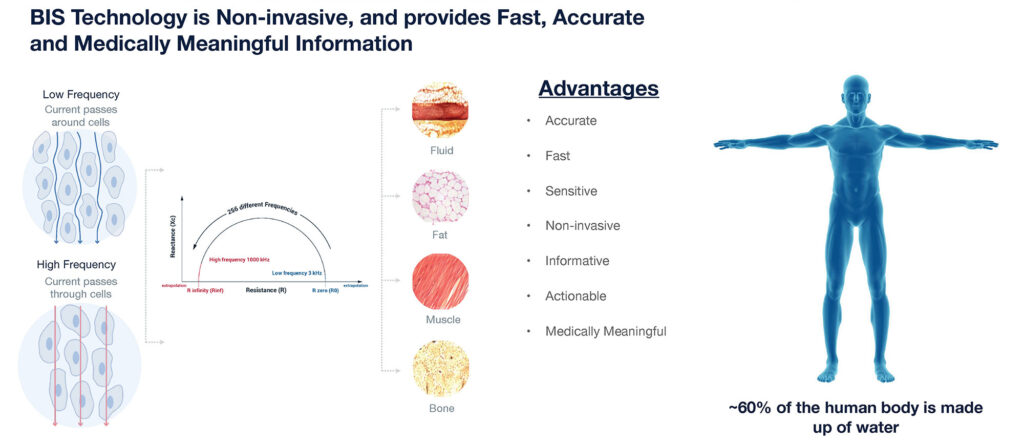
“BIS has been the subject of more than 470 peer-reviewed publications for tissue and fluid monitoring across many chronic diseases,” Mr. Carreon points out.
The company’s family of FDA cleared and CE Marked medical devices include SOZO and L-Dex. SOZO is a third-generation device for multiple indications, including lymphedema and chronic heart failure (CHF) monitoring in the clinic and at home. L-Dex measures extracellular fluid differences in the limbs for unilateral and bilateral lymphedema, which is commonly associated with removal of or damage to lymph nodes as part of cancer treatment.
Mr. Carreon says ImpediMed’s L-Dex technology is able to detect lymphedema at stage zero, up to 10 months before any physical swelling appears in a patient’s limbs. If detected early, the progression of lymphedema can be prevented and often reversed by wearing compression therapy.
“Multiple, independent, investigator-led clinical studies have reported significantly lower rates of persistent lymphedema by monitoring patients with L-Dex and [administering] compression therapy,” he adds.
Mr. Carreon explains that ImpediMed’s BIS devices apply a very low-level alternating electrical current to the body to measure impedance, or how well the body’s tissues resist current flow, at 256 electrical frequencies. Doctors testing for lymphedema, heart and renal failure, for example, are looking for buildup of a patient’s extracellular fluid.
“In less than 30 seconds, we get a detailed makeup of the fluid, fat and muscle in the body,” he adds. “And in the case of fluid, we can break that down even further into extracellular and intracellular fluid, with our devices detecting fluid shifts as little as 2 1/2 tablespoons.”
“Our technology is fast, accurate, sensitive, non-invasive, informative, actionable and medically meaningful,” Mr. Carreon contends. “We have a highly disruptive non-invasive digital health platform for the clinical monitoring of fluid and tissue.”
Mr. Carreon says SOZO has the potential to change health care by identifying early onset of disease states both in the clinic and at home, leading to improved patient outcomes.
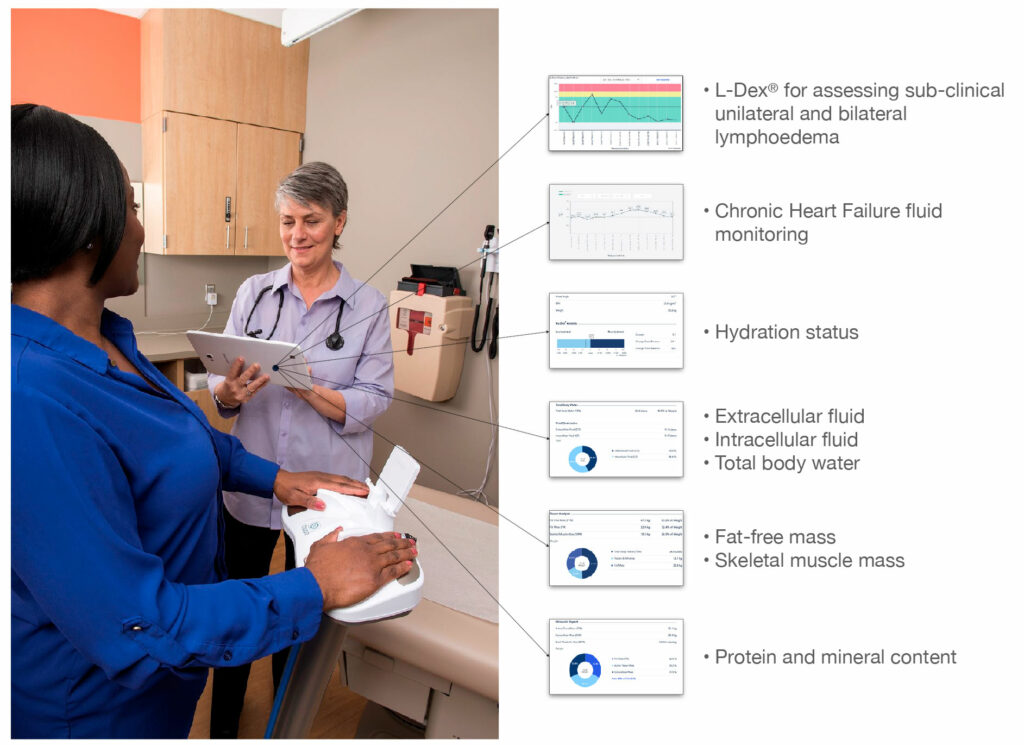
“The power of SOZO is long-term monitoring of patients so that clinicians can gain information on daily fluid changes in CHF patients and monthly or quarterly fluid changes in cancer patients.”
There are 15.5 million cancer survivors in the U.S., with 1.7 million new cases diagnosed annually. And one-in-three cancer survivors will develop lymphedema as a result of their cancer treatment, with 75% of those developing it in the first three years.
“SOZO assists with the early detection of lymphedema and could assist with other aspects of patient management, such as nutrition, bone density and muscle wasting,” Mr. Carreon contends. Cancer survivorship, following initial treatment and five-year follow up testing, represents an addressable annual market of more than $1.8-billion for ImpediMed.
In addition, cancer survivors have a 15-fold increased risk of developing CHF, which is a progressive disease where patients experience a permanent decline every time they have a major cardiac event. There are 6.5 million CHF patients in the U.S., of which 25% are classified as moderate-to-severe.
Mr. Carreon says SOZO can detect small changes in fluid levels that typically precede a major cardiac event, and that the event may be prevented by adjusting medication. Monitoring CHF failure patients represents an addressable market of more than $1-billion a year for ImpediMed.
SOZO’s worldwide customer base and adoption are accelerating, notably in large cancer centers in the U.S., because of SOZO’s ease of use and the company’s new subscription business model, he adds.
Citing the University of Kansas Cancer Center, one of four ImpediMed centers of excellence, Mr. Carreon says the institution subscribed for 14 SOZO devices last year. “The Kansas center is considering adding additional SOZO units and expanding testing to pelvic cancer patients in 2019. They expect more than 2,000 newly diagnosed cancer patients per year to be enrolled in its testing program.”
ImpediMed recently reported interim results from its PREVENT trial, a three-year follow up of 1,100 patients in the U.S. and Australia. The study is designed to determine if detection of extracellular fluid accumulation via BIS technology and subsequent early intervention can reduce the rate of lymphedema progression relative to rates where lymphedema was detected using standard tape measurements.
The primary endpoint is to achieve a relative 20% improvement over the standard of care. According to Mr. Carreon, top line results from the first year follow up of 500 patients demonstrated a 67% relative and a 10% absolute improvement in progression to persistent lymphedema in the L-Dex arm, compared to tape measurements. Second year follow up data will be reported in mid-2019.
“These interim data were medically significant,” he points out, adding that the PREVENT trial is designed to obtain additional data as a stepping-stone for reimbursement from private payers.
“We believe the PREVENT trial will establish SOZO as the standard of care for the early detection and monitoring of lymphedema in cancer survivors to either prevent the condition from developing or stop its progression.”
In CHF, Mr. Carreon says the company is initially targeting about 1.6 million class 3 patients, with the goal of providing daily monitoring either in a clinic or at home.
In a study with advanced heart failure patients that also had a CardioMEMS implant device, Mr. Carreon says SOZO BIS measurements had a correlation coefficient of 0.88 with changes in diastolic pulmonary artery pressure as measured by CardioMEMS.
“We were able to non-invasively correlate to CardioMEMS at a fraction of the $25,000 cost of an implant,” he adds.
In October 2018, ImpediMed began a study to follow some 200 CHF patients with daily measurements for 45 days at home after being discharged from hospital.
“The study will be a catalyst for initiating a broad market release of SOZO and obtaining favorable reimbursement to remotely manage patients at home,” Mr. Carreon says. “We only need to show an impact on reducing hospital readmissions and improving patient outcomes.”
ImpediMed plans to either partner SOZO in the CHF field or build its own sales and marketing team. “We have a sales staff of 12 people detailing SOZO for lymphedema, which we will continue to expand as we get closer to private payer reimbursement.”
• • • • •
To connect with ImpediMed, or any of the other companies featured on BioTuesdays, send us an email at [email protected].






